Nuvaxovid
This vaccine called Nuvaxovid produced by the company Novavax is a so. This is a multidose vial.
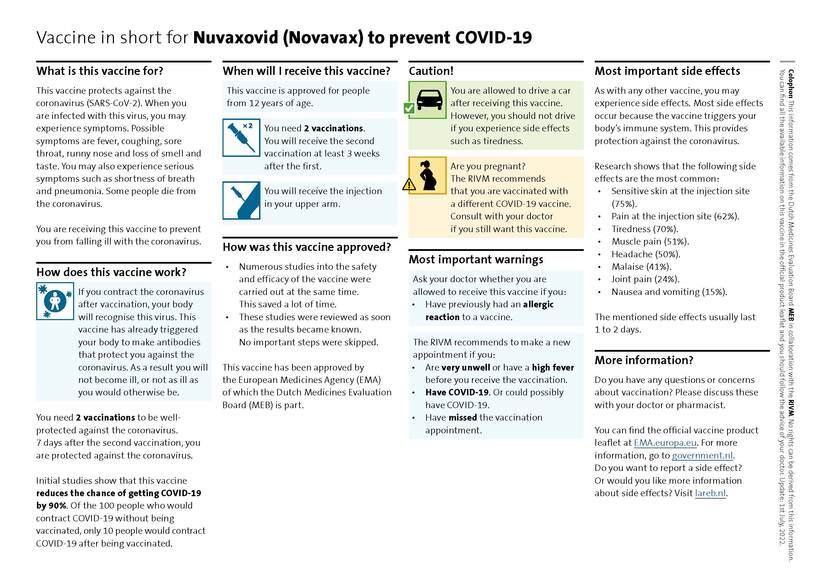
Vaccine In Short For Nuvaxovid Novavax Publication Medicines Evaluation Board
As such HSA will be monitoring the incidence rate of pericarditis or inflammation of the outer lining of the heart and myocarditis.

. Det eftersom att data från Australien gett signaler. Vaccination efforts a needed boost. Nuvaxovid contains a version of a protein found on the.
Nuvaxovid is the first protein-based COVID-19 vaccine granted authorization from the Medicines and Healthcare products Regulatory Agency MHRA and will be offered per advice from the Joint Committee on Vaccination and Immunisation. Nuvaxovid boosters could give the US. On August 19 2022 the Food and Drug Administration FDA authorized the Novavax COVID-19 Vaccine.
Nuvaxovid COVID-19 vaccines are available for use in the United Kingdom as of September 27 2022. The Technical Advisory Group for Emergency Use Listing listed Nuvaxovid NVX-CoV2373 vaccine against COVID-19 and Covovax NVX-CoV2373 vaccine against COVID-19. The Summary of Product Characteristics is a description of a.
Data från Australien pekar mot en ökad. In using an old standby technology Nuvaxovid vaccines dont have to be kept as cold as the. Novavax is approved and available for use as a booster in.
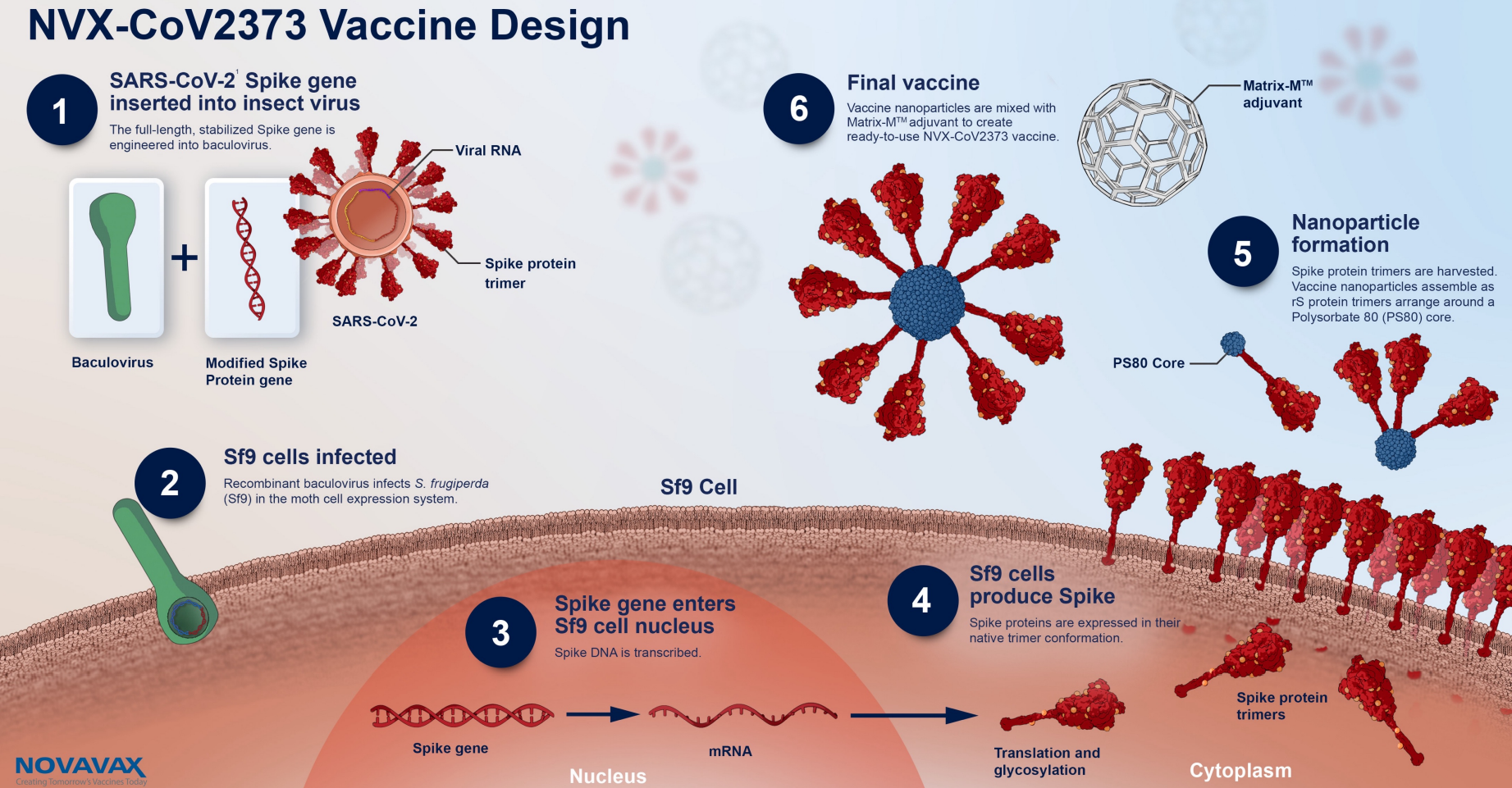
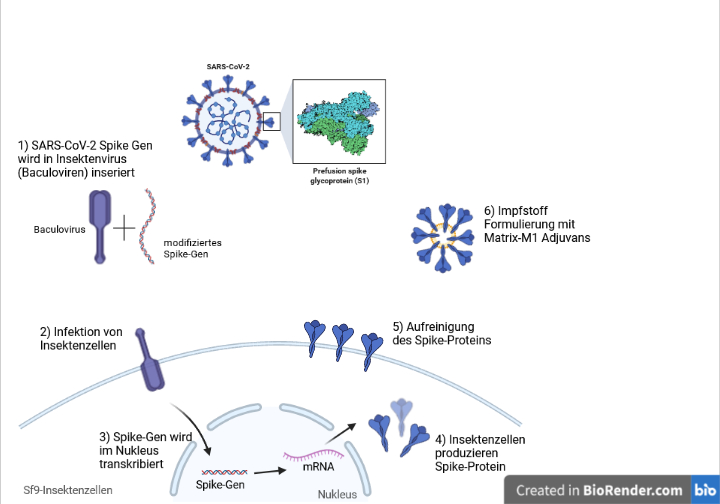
Nuvaxovid is composed of purified full length severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation. About Nuvaxovid NVX-CoV2373 Nuvaxovid is a protein-based vaccine engineered from the genetic sequence of the first strain of SARS-CoV-2 the virus that causes. The World Health Organization issued an emergency use listing EUL for Nuvaxovid TM following its assessment and approval by the European Medicines Agency.
COVID-19 Vaccine recombinant adjuvanted 2. Nu stoppar Folkhälsomyndigheten användningen bland personer som är 30. The Novavax COVID-19 vaccine sold under the brand names Nuvaxovid and Covovax among others is a subunit COVID-19 vaccine developed by Novavax and the Coalition for Epidemic Preparedness Innovations CEPI.
Nuvaxovid offers a high level of protection against COVID-19 which is a critical need in the current pandemic. After the approval of the mRNA vaccines Corminaty BiontechPfizer Spikevax Moderna and the vector-based vaccines Vaxzevria Astra Zeneca and Covid-19 Vaccine Janssen a further SARS-CoV-2 vaccine was approved by the European Medical Agency EMA in December 2021. HSAs assessment is that although the potential risk of myocarditis with Nuvaxovid COVID-19 vaccine cannot be excluded the benefits of the vaccine continue to outweigh the risks in the Singapore context it said.
Nuvaxovid is composed of purified full-length SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation. The addition of the saponin-based. The Nuvaxovid NVX-CoV2373 Novavax vaccine is a recombinant spike S protein nanoparticle vaccine combined with the Matrix-M adjuvant.
Find detailed technical information such as the product monograph and. On December 20 2021 the. Nuvaxovid dispersion for injection.
1 day agoSverige Covid-19-vaccinet Nuvaxovid skulle erbjudas till personer som var tveksamma till vaccinationen. 1 day agoBakgrunden till beslutet är signaler om ökad risk för hjärtmuskelinflammation myokardit och hjärtsäcksinflammation perikardit. Name of the medicinal product.
Company Novavax should not be given to individuals. The Novavax Nuvaxovid COVID-19 vaccine was authorized for use in Canada under the Food and Drug Regulations. Folkhälsomyndigheten rekommenderar att det proteinbaserade covid-19-vaccinet Nuvaxovid inte ges till personer som är 30 år och yngre.
1 day agoDet proteinbaserade covid-19-vaccinet Nuvaxovid ska inte ges till personer som är 30 och yngre meddelar Folkhälsomyndigheten. Qualitative and quantitative composition. Nuvaxovid Novavax is approved and available for use as a primary course in people aged 12 years and over.
Information about the COVID-19 vaccine Nuvaxovid approved by the MHRA on 03 February 2022. Full results from Nuvaxovids pivotal phase III trial were published in December 2021. Beslutet är temporärt och gäller från.
Nuvaxovid vaccine pause for young people justice system spending Västerås shooting young women have more debt than 10 years ago.

Nuvaxovid Novavax Covid 19 Vaccine Course The Immunisation Advisory Centre
Nuvaxovid Otazky A Odpovedi K Vakcine Od Spolecnosti Novavax Kurzy Cz
Switzerland Approves Its First Protein Based Covid Vaccine Swi Swissinfo Ch
U S Fda Authorizes Novavax Covid Vaccine For Adults Reuters
Racgp Tga Green Lights Nuvaxovid For 12 17 Year Olds

Investigational Vaccine Candidate Novavax Covid 19 Vaccine

Novavax S Vaccine Nuvaxovid Vaktsineeri Ee

News Nuvaxovid Novavax Protein Based Covid 19 Vaccine Available On A Limited Basis In Germany Paul Ehrlich Institut

Cdc Panel Backs Novavax Covid Vaccine For Adults Medpage Today

Vaccino Nuvaxovid Novavax Come Funziona Effetti Collaterali

Is The Novavax Covid Vaccine Worth The Hype Medpage Today

Covid 19 Vaccination About The Nuvaxovid Novavax Vaccine Youtube
Nuvaxovid The New Subunit Sars Cov 2 Vaccine Mci Innsbruck
After A Decent First Quarter Novavax S Covid Shot Is Struggling

Informacie O Covid 19 Nuvaxovid Novavax Vakcine Australian Government Department Of Health And Aged Care

Ema Recommends Nuvaxovid For Authorisation In The Eu Certifico Srl
Faq What You Need To Know About Novavax S Non Mrna Covid 19 Vaccine Nuvaxovid Cna

Ministry Of Health Singapore On Instagram Registration For The Nuvaxovid Vaccine By Novavax Has Begun Individuals Aged 18 Years And Above May Receive The Vaccine For Their Primary
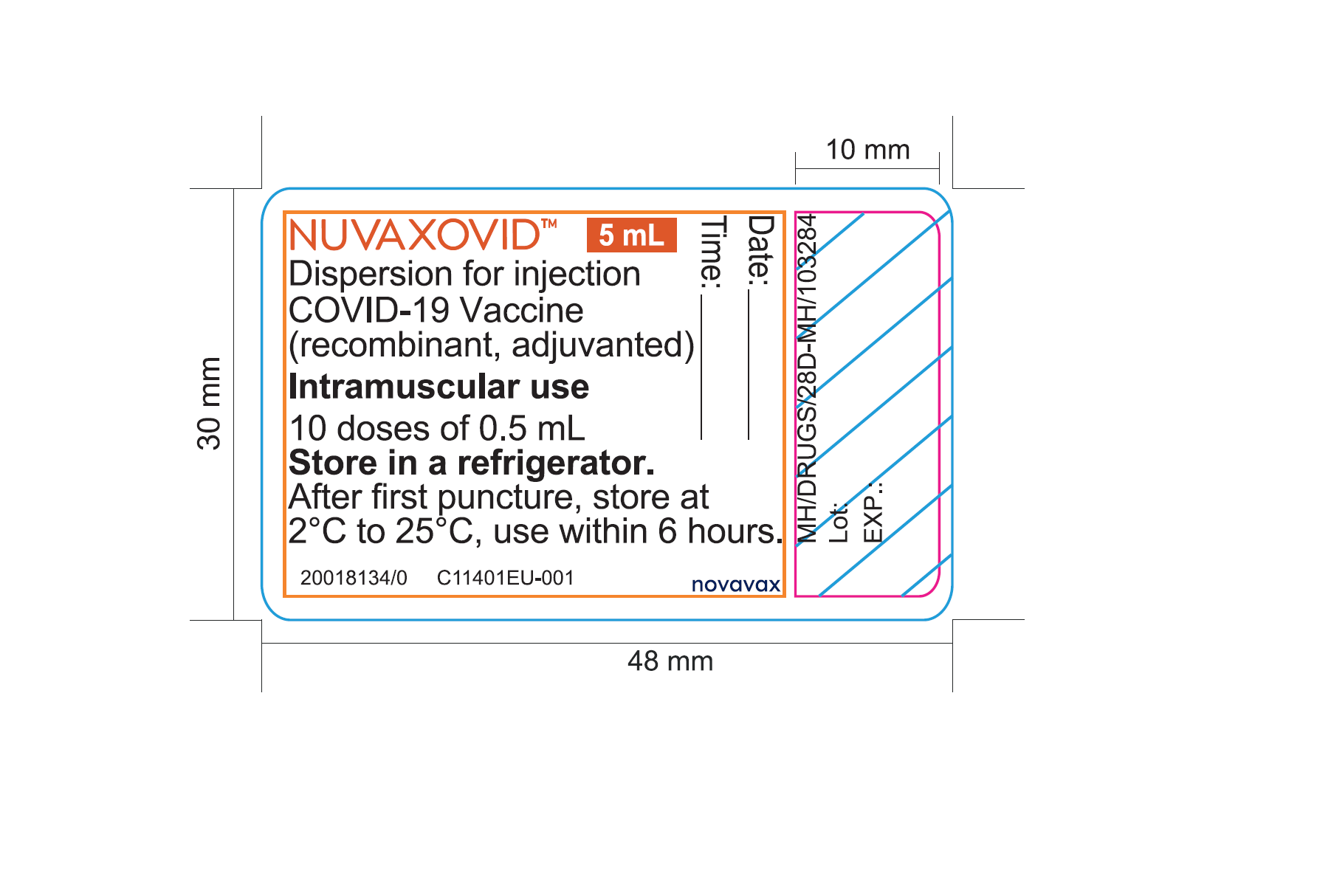
Distribution Of Nuvaxovid With English Only Vial And Carton Labels Canada Ca